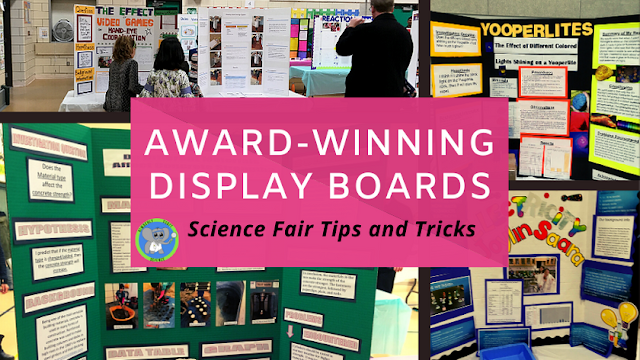
Why Does A Penny Hold So Many Drops Of Water? The Science Of A Bi-Polar Molecule.
Grab an eye dropper, a clean penny, and a cup of water. Get ready for a great surprise. Slowly add one drop of water at a time to the top of the penny. Count the number of drops until one drop spills.
How many drops did your penny hold?
Water is an amazing molecule. When water molecules are near each other they cling together forming a net-like pattern on the surface of water called surface tension. This is what creates the dome shape on top of the penny. Each water molecule is pulling another up and this creates a curved shape.
Why would water be attracted to itself?
This all has to do with positive and negative charges. Water is called a bi-polar molecule because one side is more negatively charged and the other side is more positively charged. This causes water to form a V shape. The hydrogens are at the top with a positive charge. The oxygen is at the bottom with a negative charge. When two water molecules come near each other the positive end of one water molecule attracts to the negative end of the other water molecule. We call this attraction a hydrogen bond. Think of it like the opposite ends of magnets clinging to each other. It is strong, but not so strong you can’t break it.
Each time you added a drop of water to the penny the molecules clung to each other. The drop fell and looked like it was sucked into the dome. One drop finally spills because the dome gets too tall and gravity takes hold.
Explore Further
Take this science demonstration and turn it into an experiment. What can you change? What can you measure?
In the following unit, students will do just that. They will discover surface tension and how soap affects the polar bonds of water. Students will explore with other liquids and conduct a STEM Challenge about water striders. Without water's polar bonds they could not walk across water.
Available in 3-grade level formats.
Is water wet? Can we make water wetter?
Add this reading passage and activities for a full unit.